Innate immunity is essential to maintain tissue homeostasis and is critical for vaccine and immunotherapy formulations. Because they express a variety of innate immune receptors, cells of the innate immune system such as macrophages or dendritic cells represent the first barrier against most, if not all microbes and infectious pathogens. The activation of these innate immune cells strongly relies on cellular metabolism reprogramming at the core of which is the mitochondrion, the main bioenergetic organelle. The lab investigates how host-microbe interactions control mitochondrial respiratory chain and metabolism to adjust innate immune responses to the nature of the microbe encountered. We seek at alleviating an important hurdle to vaccine design and therapeutic treatment of bacterial infections, inflammatory diseases or mitochondrial disorders.

Mitochondrial respiratory chain adaptations in host-microbe interaction
The mitochondrial electron transport chain (ETC) or respiratory chain comprises two electron carriers (coenzyme Q [CoQ]/ubiquinone and cytochrome c) and four respiratory complexes (complex I to IV [CI to CIV]), which, except for CII, can dynamically assemble as larger molecular supercomplexes (SCs) in the mitochondrial inner membrane. This dynamic assembly of respiratory complexes into SCs has been proposed to confer functional advantages to the cells but its relevance for innate immunity is still unknown. Here, we aim at providing the molecular details that govern the ETC and mitochondrial metabolic adjustments evoked during antibacterial immunity. We pay a particular attention to the contribution of innate immune receptors such as the Toll-like receptors and the cyclic GMP-AMP synthase (cGAS) in regulating these processes.
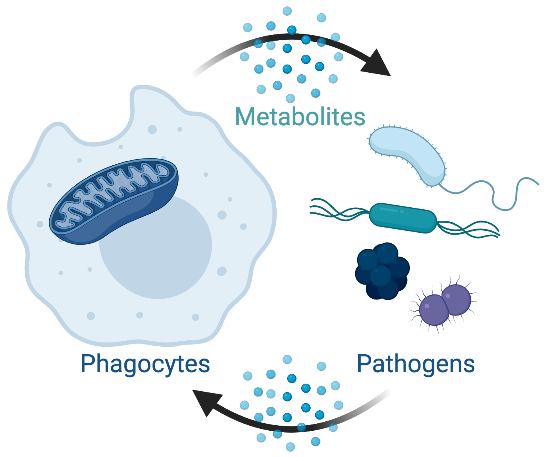
Fueling innate immune cell metabolism with microbes
As all cells in the organism, phagocytes use nutrients from the microenvironment to fuel their metabolic and bioenergetic needs. Yet, they possess the unique capacity to engulf multiple microbes. This creates an acute situation of nutrient overload that needs to be resolved and may influence metabolic reprogramming. Here, we are investigating the molecular determinants that allow innate immune cell metabolism to integrate the metabolic features and the ‘nutritive potential’ of microbes and how this sets the nature of the immune response.
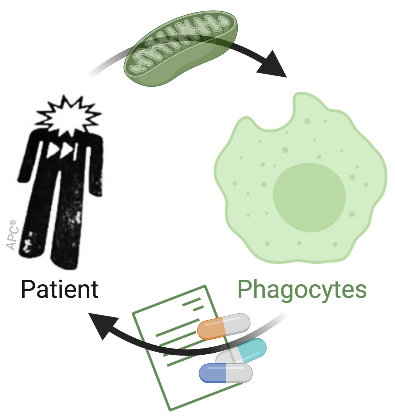
Mitochondrial disease-associated immune dysfunctions
Mitochondrial and cellular metabolism reprogramming is central to immunity. Yet, immune consequences of mitochondrial dysfunctions are largely unknown in human. Strikingly, immune dysfunction is not currently included in clinical diagnostic criteria for mitochondrial disorders although remarkable rates of infections have been noted in cohorts from two primary mitochondrial diseases. Using patient samples, we assess the impact of mutations in the mitochondrial DNA (mtDNA) on innate immunity, anti-microbial responses and antigen presentation. Our objective is to provide clinically-relevant information on the immune consequences of mitochondrial diseases to alleviate hospitalization and disease burden.

